Heart failure is a complex pathological process is that is initiated by an index event that injures the heart. Examples can be divided into ischemic (myocardial infarction) or nonischemic (viral infection, exposure to chemotherapy, genetic cardiomyopathies) etiologies. Outside of coronary revascularization for ischemic heart disease, no therapies yet exist to reverse the primary causes of heart failure.
Patients diagnosed with heart failure receive a combination of medications that target a processed referred to as adverse remodeling, which results in progressive cardiomyocyte loss, fibrosis, hypertrophy, and dilation of the heart. Despite these interventions, the majority (>80%) of patients undergo disease progression and develop advanced heart failure. However, a minority of patients (<20%) will experience recovery of cardiac function and reversal of their disease. While these observations suggest that it is possible to repair the failing heart, little is known regarding the mechanisms that dictate whether a patient will recover or experience disease progression.
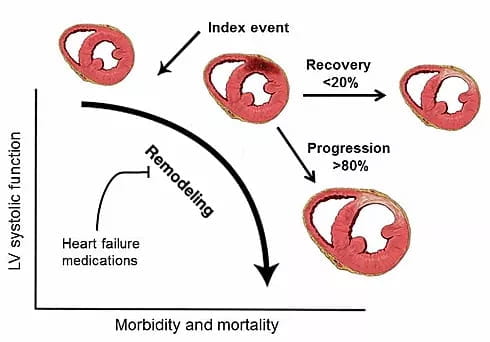
Change in LV systolic function as a function of mortality and morbidity. As >80% of patients continue into heart failure progression compared to <20% of patients who have LV recovery, loss of cardiomyocytes, fibrosis, and hypertrophy occur.
The primary focus of our laboratory is identify new treatments that reverse the primary causes of heart failure and improve the heart’s intrinsic ability to undergo tissue repair.
Precision Therapeutics for Dilated Cardiomyopathy
The vast majority of patients with dilated cardiomyopathy carry genetic mutations in sarcomeric, cytoskeletal, or mitochondrial genes. However, it is not clear why these mutations result in heart failure.
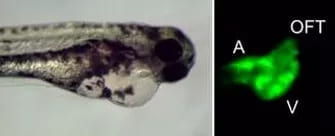
We use Zebrafish as a model system and have inserted established and novel dilated cardiomyopathy mutations into the genome using Cas9/CRISPR technology to define mutation specific disease mechanisms, identify precision therapies, and explore genetic interactions between these variants.
Macrophage Heterogeneity
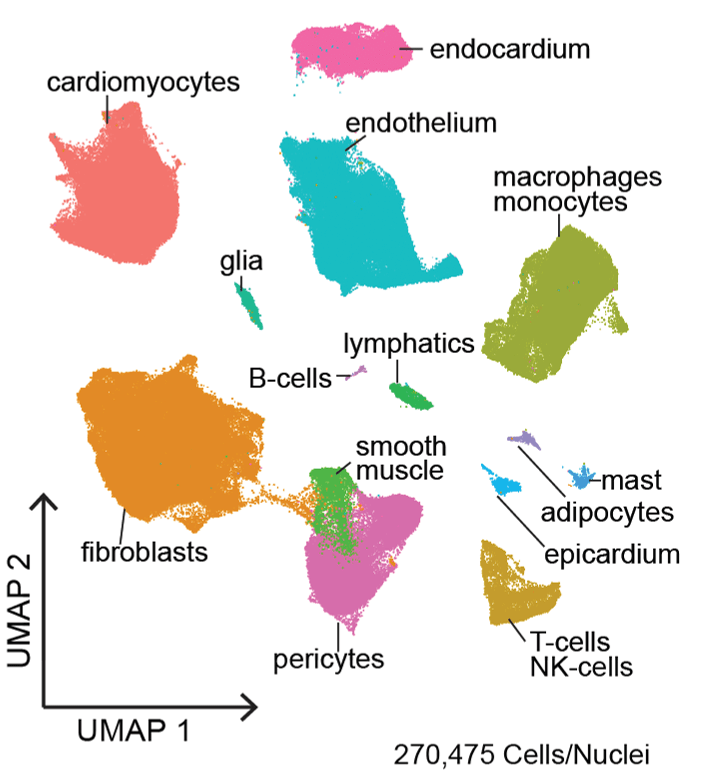
Activation of the immune system is common mechanism by which the heart responds to injury. Within the heart, macrophages represent the most prevalent immune cell. By carefully examining macrophage composition within the heart under homeostatic and disease conditions, we discovered that the myocardium contains a diverse array of macrophage subsets derived from divergent origins each with unique reparative and inflammatory functions.
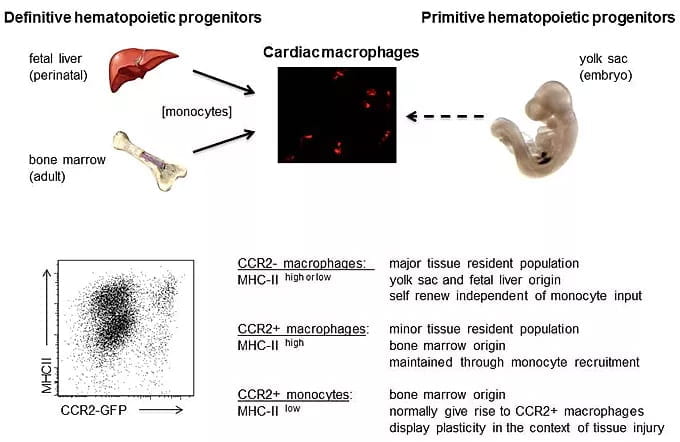
We are interested in determining whether the human heart contains similar macrophage subsets, defining signaling pathways by which each tissue resident and recruited subset is activated and contributes to heart failure progression and/or tissue repair, and deciphering whether other cardiac cell types display a similar degree of diversity.
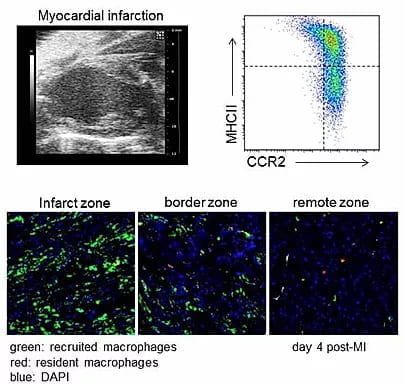
Macrophage ontogeny shifts and monocyte fate decisions. Following ischemic cardiac injury a dramatic shift in macrophage composition occurs, where resident macrophages derived from embryonic yolk sac and fetal liver progenitors are replaced by recruited macrophages derived from adult bone marrow monocytes. An important consequence of this ontogeny shift is the accumulation of inflammatory CCR2+ monocytes and macrophages at the expense of reparative macrophage subsets. Collectively, CCR2+ monocytes and macrophages function to drive adverse remodeling and heart failure progression through the generation of inflammatory cytokines and oxidative products that promote collateral tissue injury.
Current questions in the laboratory are focused on how resident macrophages subsets deferentially orchestrate ontogeny shifts and why recruited monocytes preferentially acquire an inflammatory fate. To address these questions, we utilize a combination of myocardial infarction, genetic lineage tracing, parabiosis, and heart transplant models to differentiate between resident and recruited immune subsets.
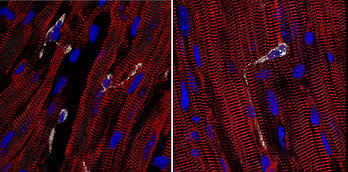
Novel roles for tissue resident macrophages in the heart. By characterizing mouse models of dilated and ischemic cardiomyopathy, we have uncovered unexpected functions of cardiac tissue resident macrophages. Examples include facilitation of electrical conduction, detection of mechanical strain, and cell junction formation. We have delineated some of the the molecular mechanisms that regulate how macrophages orchestrate these activities.
Applications to regenerative medicine. Using human pluripotent stem cells, we have developed methodologies to generate macrophages derived from primitive and definitive hematopoiesis. These macrophages share characteristics of resident CCR2- cardiac macrophages and display robust reparative potential.
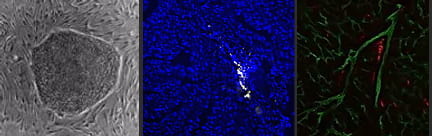
Single Cell RNA Sequencing in the Heart
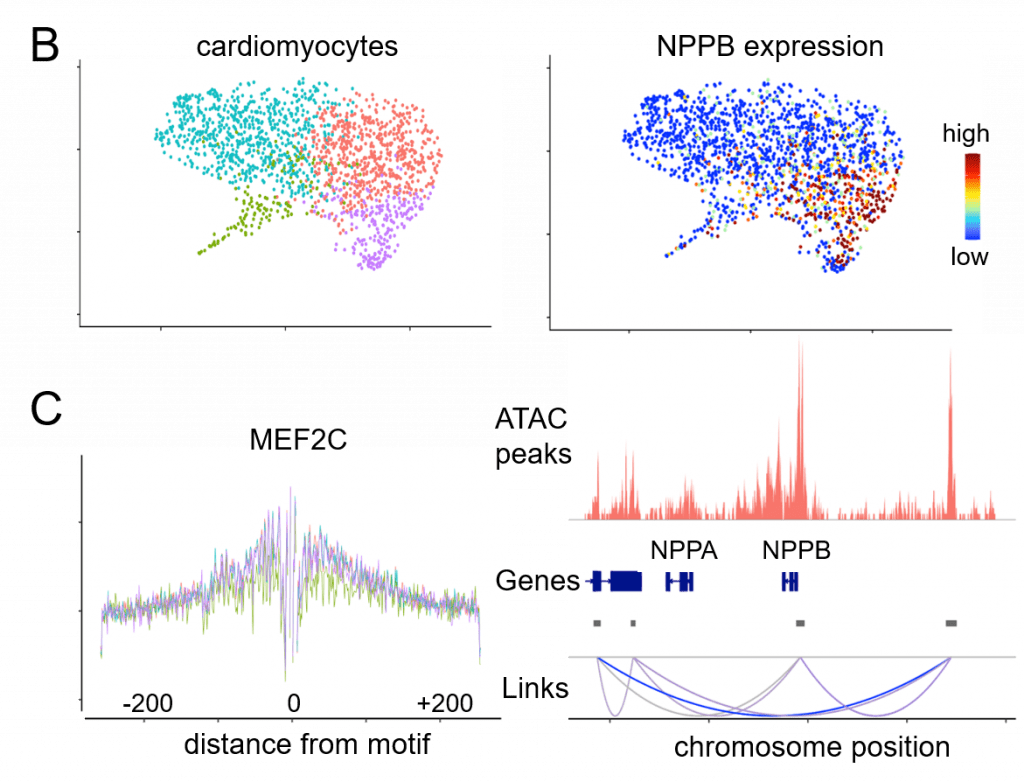
Current projects are focused on understanding the requirements for primitive and definitive macrophage specification and delineating whether these cells can be used to improve cardiac tissue regeneration. Additionally, projects involving single cell RNA sequencing techniques on left ventricular myocardium, coronary arteries, and aortic and mitral valves are taking place to understand better the various unique cell populations that exist in patients with advanced heart failure.